
Our experienced staff is a valuable resource, offering a variety of support and oversight throughout the duration of your study. The Canfield team thrives on your problem and creating solutions that best fit your needs. We have study experience ranging from small to large, early phase to marketing and are able to adapt our solutions accordingly.

Study Design Consultation
With over 30 years of experience, our team will collaborate with you to establish the imaging methodology tailored to your protocol. We have the insight and knowledge to ensure the best in class products and services are provided for your needs.
Our Quality Assurance team oversees study deliverables and ensures our Standard Operating Procedures (SOPs) are followed and regulatory compliance is achieved.

Site Training and Support
Our goal is to keep the photography process simple and efficient. We provide on-site installation and training, attend Investigator Meetings, host web based trainings and assist with remote support as needed. The success of your trial is based on the success of the study sites and we work closely with the study sites to ensure this success. Our teams are available around the world and remotely to provide support to the study sites as needed.
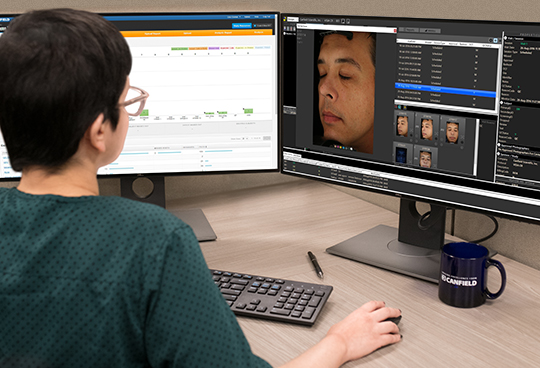
Image Review
With experience in thousands of clinical trials, our team is properly trained and qualified to monitor study images and confirm imaging protocol compliance. We strive to ensure the highest quality and consistency is followed by the Investigator Sites. Success is achieved by keeping in close communication with both the investigative sites and the sponsor.



 Steven Kempers, MD,
Steven Kempers, MD, Robert Weiss, MD,
Robert Weiss, MD, Jason D. Bloom, MD,
Jason D. Bloom, MD,